Chemical Properties of Carboxylic Acids and its Derivatives
Chemical Properties of Carboxylic Acids and its Derivatives: Overview
This topic covers concepts such as Chemical Properties of Carboxylic Acids, Reactions of Carboxylic Acids involving Cleavage of O-H Bond, Acidity of Carboxylic Acids, and Reaction of Carboxylic Acids with Sodium.
Important Questions on Chemical Properties of Carboxylic Acids and its Derivatives
The correct increasing order of acidic strength of the following compounds is
benzoic acid
nitro benzoic acid
, dinitrobenzoic acid
methoxybenzoic acid
The reagents that can be used to distinguish between the following pairs of compounds would be:
(i) Propanal and Propanone
(ii) Phenol and Benzoic acid
Acetyl bromide reacts with excess of followed by treatment with a saturated solution of gives –
Among the following acids which has the lowest value?
A liquid was mixed with ethanol and a drop of concentrated H2SO4 was added. A compound with a fruity smell was formed. The liquid was:
A liquid was mixed with ethanol and a drop of concentrated H2SO4 was added. A compound with a fruity smell was formed. The liquid was:
Which amongst the following compounds has the highest acid strength?
Among the following the lowest value is of
Choose the reagents used to transfer carboxylic acid to acid chloride.
Which of the following reagents can convert acid chloride into alcohols?
Consider the reaction.
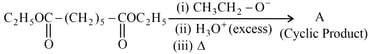
The functional group of cyclic compound (A) is:
The self-condensation reaction of one molecule of -hydroxyhexanoic acid gives
The Hell-Volhard-Zelinsky reaction is used to
When chlorine is passed through acetic acid in presence of halogen carrier (red ), it forms
Write the equation for the alkylation of carboxylic acids?
Explain the mechanism of alkylation of carboxylic acids?
At what position of carboxylic acids does alkylation take place?
Identify the reagents and in the following reaction
In which one of the following cases will the -hydrogen NOT be abstracted on treatment with one equivalent of base?
Effervescence takes place when sodium carbonate solution is added to
